Abstract
Background: Epcoritamab, a subcutaneously administered (SC) bispecific CD3xCD20 antibody, has shown potent antitumor activity as a single agent and in combination. Results of the EPCORE NHL-1 LBCL expansion cohort demonstrated clinically meaningful and compelling efficacy across a highly refractory population and a manageable safety profile, consistent with prior findings (Thieblemont et al, EHA 2022). Here, we present additional subgroup data on the efficacy and safety of epcoritamab in the LBCL expansion cohort of the EPCORE NHL-1 phase 2 trial (NCT03625037).
Methods: Adults with R/R CD20+ LBCL were treated with 1-mL SC priming and intermediate doses, followed by full doses of epcoritamab in 28-d cycles (QW: cycles 1-3; Q2W: cycles 4-9; Q4W: cycles ≥10). Step-up dosing and corticosteroid prophylaxis were administered in cycle 1 to mitigate CRS. Prespecified and exploratory subanalyses were performed; response was assessed by independent review committee.
Results: As of Jan 31, 2022, 157 patients (pts) with LBCL, including double/triple-hit (DH/TH) lymphoma, were treated. Per protocol there was no minimum life expectancy requirement. Median age was 64 y (range, 20-83), median number of prior lines of therapy was 3 (range, 2-11), and median time from initial diagnosis was 1.6 y. Overall, 39% of pts had received prior CAR T, 20% had prior autologous stem cell transplantation, and 61% had primary refractory disease; 14% of pts with diffuse LBCL (DLBCL; 12/88 tested) were confirmed DH/TH by central FISH.
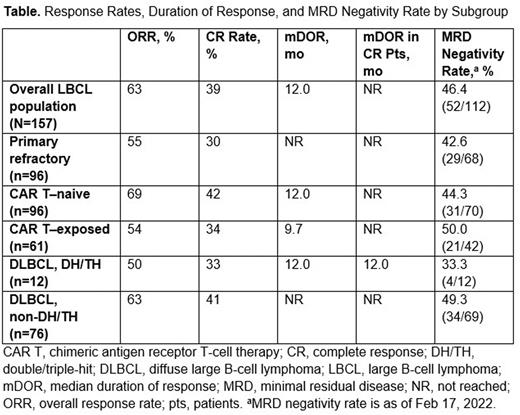
At a median follow-up of 10.7 mo, the overall response rate (ORR) for the total population was 63%, with a complete response (CR) rate of 39%. The median duration of response (mDOR) was 12.0 mo (95% CI, 6.6-not reached [NR]) overall. In subgroup analyses, mDOR was NR (95% CI, 2.8-NR) for primary refractory pts, 9.7 mo (95% CI, 5.4-NR) for pts with prior CAR T, 12.0 mo (95% CI, 5.6-NR) for CAR T-naive pts, and 12.0 mo (95% CI, 1.1-NR) for DH/TH pts. Responses occurred early, with a median time to response of 1.4 mo. The mDOR in pts who achieved a CR was 12 mo for DH/TH pts and not reached in other subgroups. MRD negativity was assessed by a ctDNA NGS assay in this LBCL population with 52 of 112 evaluable pts (46.4%) being MRD negative at any time point on treatment. MRD negativity was reached early (median of 8 wk for complete responders), and high MRD negativity rates were observed across all subpopulations: 50% in pts with prior CAR T, 44% in CAR T-naive pts, 33% in DH/TH pts, and 49% in non-DH/TH pts. MRD-negative responses were durable (Table) and correlated with PFS. Durability of CR was also demonstrated in a cohort of pts with DLBCL from the dose-escalation portion of this trial with extended follow-up (median, 21.1 mo; longest follow-up, 26.7 mo), of which 6 pts (at ≥12 mg) remain on Q4W treatment and in CR, with median duration of CR not reached.
Overall, epcoritamab was well tolerated. Incidence of AEs declined after wk 12, and only 1 pt experienced any related serious treatment-emergent AE after wk 12 (CRS grade 1/2). CRS was reported in 78 pts (49.7%), and all but 1 event resolved. Events were primarily low grade (31.8% grade 1, 15.3% grade 2, and 2.5% grade 3), and timing was predictable, with 5.7% of pts experiencing CRS after the priming dose, 11.8% after the intermediate dose, 42.9% after the first full dose, 4.9% after the second full dose, and 2.9% after the third full dose or later doses. Median time to CRS onset after the first full dose was 20.0 h (range, 12-126). Ten pts had ICANS events; 9 events were grade 1 or 2, and there was 1 grade 5 event (the only treatment-related death, pt with multiple confounding factors). All but 1 ICANS event occurred in cycle 1. There were 8 pts (5%) who experienced both CRS and ICANS. Grade 3 or 4 neutropenia occurred in 33 pts (21.0%; 10.8% grade 3, 10.2% grade 4). Grade 3 anemia was reported in 16 pts (10.2%). Few pts discontinued epcoritamab treatment due to AEs (n=11). Notably, there were no treatment discontinuations due to neutropenia or anemia.
Conclusions: SC epcoritamab is a convenient, off-the-shelf therapy that has demonstrated deep and durable responses including high MRD negativity rates as a single agent. The safety profile was favorable, and CRS events were mostly low grade and transient, with most events occurring after the first full dose. These subgroup analyses demonstrate consistent, clinically meaningful activity of epcoritamab across subgroups of pts with R/R LBCL.
Disclosures
Phillips:BMS: Consultancy, Research Funding; Eli Lilly: Consultancy; Epizyme: Consultancy; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Consultancy; Kite/Gilead: Consultancy; Pharmacyclics/Janssen: Honoraria; ADCT: Consultancy; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Research Funding; Beigene: Consultancy; Abbvie: Consultancy, Research Funding; Lymphoma & Myeloma Connect: Honoraria; Celgene: Consultancy; Incyte: Consultancy; Xencor: Consultancy; Curis: Consultancy; TG Therapeutics: Consultancy. Thieblemont:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Hospira: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding. Ghesquieres:BMS: Honoraria; Gilead: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Abbvie: Honoraria. Cheah:BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Ascentage Pharma: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: Travel expenses, Research Funding. Cunningham:Celgene: Research Funding; Bayer: Research Funding; 4SC: Research Funding; Lilly: Research Funding; Clovis: Research Funding; AstraZeneca: Research Funding; MedImmune: Research Funding; Roche: Research Funding. Gasiorowski:MSD: Honoraria; Novartis: Honoraria; Antengene: Honoraria; Astellas: Honoraria; Otsuka: Honoraria; Janssen: Honoraria; AbbVie: Honoraria. Jurczak:Mei Pharma: Research Funding; Lilly: Consultancy, Research Funding; Takeda: Research Funding; AbbVie: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Bayer: Research Funding; Celgene: Research Funding; Roche: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; TG Therapeutics: Research Funding; Loxo Oncology: Consultancy, Research Funding; Sandoz: Consultancy, Research Funding; Merck: Research Funding; Morphosys: Research Funding; Novo Nordisk: Research Funding. Kim:Regeneron: Other: Clinical trial research funding to my institution; Merck Sharp & Dohme: Other: Clinical trial research funding to my institution; Merck Serono: Other: Clinical trial research funding to my institution; Genmab: Other: Clinical trial research funding to my institution; Boryung: Other: Clinical trial research funding to my institution; Celgene: Other: Clinical trial research funding to my institution; Boehringer-Ingelheim: Other: Clinical trial research funding to my institution; BMS: Other: Clinical trial research funding to my institution; Bayer: Other: Clinical trial research funding to my institution; AstraZeneca/MedImmune: Other: Clinical trial research funding to my institution; Takeda: Honoraria, Other: Clinical trial research funding to my institution; Roche/Genentech: Honoraria, Other: Clinical trial research funding to my institution; Novartis: Honoraria, Other: Clinical trial research funding to my institution; Hanmi: Honoraria, Other: Clinical trial research funding to my institution; Janssen: Honoraria, Other: Clinical trial research funding to my institution; AstraZeneca: Honoraria; AstraZeneca-KHIDI: Research Funding; Sanofi: Other: Clinical trial research funding to my institution. Lewis:Beigene: Consultancy; Kite: Consultancy, Other: Travel grant; Janssen: Consultancy, Other: Speakers fees; travel grant; Roche: Consultancy; Eli Lilly and Company: Consultancy. Kilavuz:Genmab: Current Employment. Cota Stirner:AbbVie: Current Employment. Soong:Genmab: Current Employment. Chiu:Genmab: Current Employment, Current equity holder in publicly-traded company. Chen:Genmab: Current Employment. Sacchi:Genmab: Current Employment, Current equity holder in publicly-traded company. Elliot:Genmab: Current Employment, Current equity holder in publicly-traded company. Hutchings:AbbVie: Consultancy; Genmab: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Incyte: Research Funding; Novartis: Research Funding; Genentech: Research Funding; Celgene, Genentech, Genmab, Incyte, Janssen, Novartis, Roche, Takeda: Research Funding; AbbVie, Celgene, Genmab, Janssen, Roche, Takeda: Membership on an entity's Board of Directors or advisory committees. Lugtenburg:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal